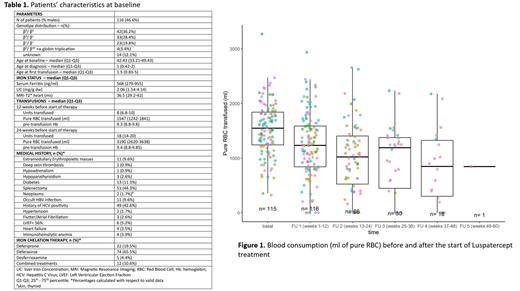
Luspatercept has been demonstrated to reduce the need for blood transfusion with manageable side effects in clinical trials, thus receiving the approval for the treatment of anemia associated with transfusion dependent beta thalassemia in adults. However, very little data on its safety and efficacy in real life have been hitherto published. Special warnings and precautions for use refer to the possibility of occurrence of thromboembolic events, extramedullary hemopoiesis masses and increased blood pressure. Ten Italian Specialized Centres for the treatment of Hemoglobinopathies, with the patronage of the “Società Italiana Talassemie ed Emoglobinopatie” (SITE), evaluated the effects of luspatercept in terms of safety and blood transfusion reduction in 116 patients (Table 1) who received the first dose of the drug after its marketing. The median duration of treatment was 158 days (min-max: 15-442). β globin genotype was β 0/β 0 in 42 subjects (36.2%), β 0/ β + in 33 (28.4%), β +/ β + in 23 (19.8%), β 0/β wt plus α gene triplication in 4 (3.4%) and unknown in 14 (12%). Sixty-four patients (55.2%) were splenectomized. At the time of data collection, 32 patients (27.6%) had already discontinued the drug. Discontinuation had occurred after a median time of 132 days of treatment (min-max: 15-338) due to insufficient efficacy in the judgement of the physician (10) or patient (3), side effects attributed to luspatercept (16) that were detrimental to the continuation of therapy in the judgement of the physician (6) or patient (10), pregnancy (1) and other causes (2).
There were 13 patients with a transfusion-free interval of at least 8 weeks during the treatment period (median 12 weeks, min-max range 9-48). Their median age at baseline was 37.6 years (min-max range 18.4 - 51.6) and age at first transfusion 24 years (min-max range 1 - 33). β globin genotype was β 0/ β 0 in 1 (7.7 %), β 0/ β + in 2 (15.4 %), β +/ β + in 7 (53.8 %), β 0/ β wt plus α gene triplication in 3 (23.1 %). Patients who decreased transfusion requirements by ≥ 33% and ≥ 50% in at least one of the follow-up periods of 12-weeks were 52 (44.8%) and 36 (31%), respectively. When considering the 13-24 week interval (considered as the primary endpoint of the Believe study), of the 66 patients who completed the second follow-up, 31 of 66 patients (47.0%) reduced their transfusion load by ≥33% and 20 of 66 patients (30%) by ≥50%. Focusing on the first two quarters of treatment, the pairwise t test showed a progressive and significant (p<0.05) reduction in the number of transfused units and pure red blood cell volumes (figure). There were no significant differences between the pre-treatment hemoglobin value and that during treatment.
Serum ferritin also decreased progressively (p<0.05) during treatment (basal: median 576 ng/mL (Q1-Q3: 289-992); weeks 1-12: 479 ng/mL (232- 848); weeks 13-24: 385 ng/mL (212-644).
The pregnancy that was initiated during the luspatercept treatment is still ongoing and no definitive information can be given.
Sixty patients (51.7%) reported at least one side effect potentially related to luspatercept. The most frequent side effects were bone pain (40), asthenia-fatigue (11), hypertension requiring therapy or modification of therapy (11), joint pain (11), folic acid deficiency (6), heart palpitations (4) and edema (4). In two patients, an asymptomatic increased volume of masses of extramedullary erythropoiesis was reported. Another two patients presented with thromboembolic events: ischemic stroke in one case and superficial venous thrombosis in the other. Both were splenectomized with no other known risk factors.
Despite the limitations of the short duration of follow-up, the analysis reports several significant results. The high percentage of patients who decided not to continue treatment underlines the need for a proper management of patient expectations and selection.
The drug proves to be effective also in real life and those who respond best in the short and medium term seem to be who already have residual erythropoiesis, as seen from the age at the start of transfusions and the genotype of those who have had a long period of transfusion independence. Although the drug is generally well-tolerated, some adverse events appear more frequent than reported in Believe. Finally, the report of the two patients who developed a thromboembolic event including stroke, suggests the importance of continuing to be cautious in this regard.
Disclosures
Lisi:BMS: Honoraria. Zappu:Bristol Myers Squibb: Speakers Bureau. Motta:BMS: Consultancy, Speakers Bureau; Sanofi Genzyme: Consultancy, Honoraria, Research Funding, Speakers Bureau; Amicus Therapeutics: Consultancy. Longo:Vertex: Membership on an entity's Board of Directors or advisory committees; Bristol Meyer Squibb (BMS): Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Pinto:Novartis: Speakers Bureau; Vertex: Membership on an entity's Board of Directors or advisory committees; Bluebirdbio: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. De Franceschi:Bristol Myers Squibb: Research Funding; Agios: Research Funding; F. Hoffmann-La Roche Ltd, Basel: Membership on an entity's Board of Directors or advisory committees. Ruffo:Vertex: Consultancy; BMS: Speakers Bureau.